-- Study evaluates accuracy of CPS compared to gold-standard right heart catheterization to support FDA submission --
Sensydia, a medical technology company pioneering non-invasive cardiac assessment, today announced that the first patient has been enrolled in its multi-center pivotal study to evaluate the accuracy of the Cardiac Performance System (CPS) for hemodynamic assessment as compared to the gold-standard: invasive right heart catheterization.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250428025023/en/
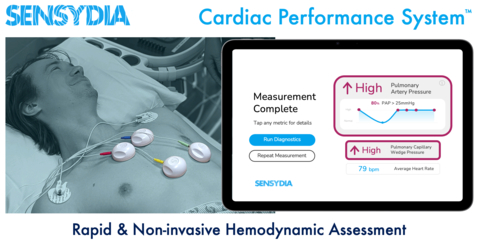
Sensydia’s Cardiac Performance System (CPS™) is designed for fast, safe, and non-invasive cardiac performance assessment that can be performed almost anywhere, avoiding the need to visit a catheterization lab. Source: Sensydia.
Sensydia’s CPS acquires cardiovascular signals, including heart sounds, and analyzes them using artificial intelligence algorithms, to provide clinicians a non-invasive tool for earlier assessment of cardiac function and to help them more effectively guide therapy for patients suffering from heart failure and pulmonary hypertension. The current practice to obtain these measurements is for patients to undergo echocardiography and invasive right heart catheterization, which are resource intensive, restricted to medical facilities, and only provide snapshot data. In contrast, CPS assessments are fast, safe, may be repeated as frequently as needed, and can be performed in the clinic with minimal training.
Previous studies have demonstrated the potential of CPS to accurately estimate pulmonary pressure, suggesting its utility in clinical practice. This multi-center observational study will evaluate CPS's accuracy compared to the gold-standard invasive right heart catheterization in measuring hemodynamic parameters. The study’s outcomes will support Sensydia's efforts toward FDA submission and commercialization of CPS.
"Enrolling the first patient in our pivotal study marks a significant milestone for Sensydia as we advance our mission to transform cardiac care," said Anthony Arnold, President and CEO of Sensydia. "We believe CPS has the potential to provide clinicians with critical hemodynamic information without the risks associated with invasive procedures."
"The ability to obtain accurate hemodynamic assessments non-invasively will significantly impact how we manage patients with heart failure and pulmonary hypertension," said James D. Murphy, MD, FACC, Principal Investigator at the first site – Huntsville Hospital Heart Center in Alabama. "We are excited to participate in this important study evaluating CPS."
Please note: CPS is undergoing a clinical study and is not yet FDA-approved.
About Sensydia
Sensydia is developing the Cardiac Performance System (CPS™), a non-invasive platform that provides real-time measurements of critical cardiac function. CPS is designed to deliver rapid, safe, and accurate assessments to improve outcomes for patients with heart failure and pulmonary hypertension. The company received FDA 510(k) clearance for non-invasive measurement of ejection fraction using first-generation hardware in 2018. Learn more at sensydia.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250428025023/en/
Contacts
Media:
Kathryn Morris, BrightPoint
kathryn@brightpointny.com
914-204-6412
